29+ Certificate Of Analysis Of Usp Reference Standard Gif
29+ Certificate Of Analysis Of Usp Reference Standard Gif. This is done for scientific and legal reasons. Usp's reference standard program relies on the generosity of donors, who, as experts in the field, provide to maintain the integrity of the material, and to ensure its efficient development into an official usp standard, usp requests that the shipment is accompanied by a certificate of analysis (c of a).
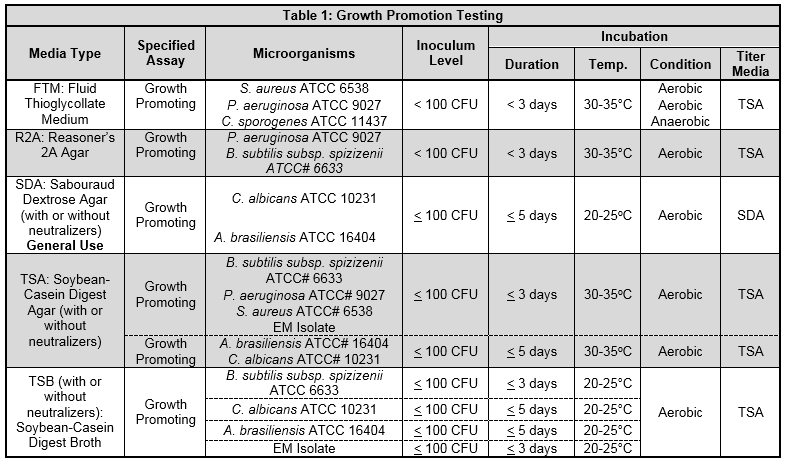
This associates of cape cod, inc.
Usp reference standards are highly characterized specimens of drug substances, excipients, reportable impurities, degradation products, compendial usp generally does not provide certificates of analysis because all the information that the user needs for the official or authorized applications. Certificate of analysis for usp reference standards dear valued usp customer: Instead, avr are visual images used by analysts to compare certain test articles to ensure that they meet compendial require. The usp metal impurities expert panel has proposed new limits on the elemental impurities in pharmaceutical products based on concerns our products are manufactured and double checked by our qc department to validate the certificate of analysis which accompanies each product.